
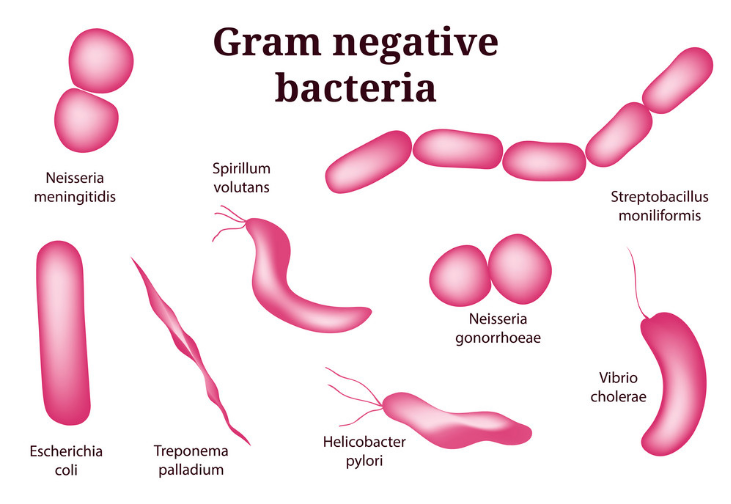

Guidance on preventing healthcare associated Gram-negative bacterial bloodstream infections is available from the NHS Improvement website. Some of the antibiotic resistance mechanisms are on mobile genetic elements, such as plasmids, which allow the genes that encode resistance to spread more easily, and importantly, between different bacterial species. Gram-negative bacteria can be resistant to antibiotics and in some cases will be multi-resistant rendering most available antibiotics useless. and Pseudomonas aeruginosa are the leading causes of healthcare associated bloodstream infections. Gram-negative bacteria such as Escherichia coli, Klebsiella spp. One of the most serious infections Gram-negatives can cause bloodstream infections. Some live in the intestine harmlessly, while others may cause a variety of diseases.īacteria that are normally harmless in their normal environment can cause problems if they grow in other parts of the body and can cause a range of infections with differing severity and associated mortality. Changing the use of tetracyclines in human and animal health as well as in food production is needed if we are to continue to use this class of broad-spectrum antimicrobials through the present century.There are many different types of Gram-negative bacteria. New tetracycline derivatives are being examined, although their role in treatment is not clear. A limited number of bacteria acquire resistance by mutations, which alter the permeability of the outer membrane porins and/or lipopolysaccharides in the outer membrane, change the regulation of innate efflux systems, or alter the 16S rRNA. Many of these genes are associated with mobile plasmids or transposons and can be distinguished from each other using molecular methods including DNA-DNA hybridization with oligonucleotide probes and DNA sequencing.
#Gram negative bacteria code#
Tetracycline resistance is often due to the acquisition of new genes, which code for energy-dependent efflux of tetracyclines or for a protein that protects bacterial ribosomes from the action of tetracyclines. The presence of tetracycline-resistant pathogens limits the use of these agents in treatment of disease. Tetracycline resistance now occurs in an increasing number of pathogenic, opportunistic, and commensal bacteria. The first tetracycline-resistant bacterium, Shigella dysenteriae, was isolated in 1953. They are inexpensive antibiotics, which have been used extensively in the prophlylaxis and therapy of human and animal infections and also at subtherapeutic levels in animal feed as growth promoters. These nontoxic nanomaterials, which can be prepared in a simple and cost-effective manner, may be suitable for the formulation of new types of bactericidal materials.Ībstract: Tetracyclines were discovered in the 1940s and exhibited activity against a wide range of microorganisms including gram-positive and gram-negative bacteria, chlamydiae, mycoplasmas, rickettsiae, and protozoan parasites. A membrane with such a morphology exhibits a significant increase in permeability, resulting in death of the cell. coli cells were damaged, showing formation of "pits" in the cell wall of the bacteria, while the silver nanoparticles were found to accumulate in the bacterial membrane. The results confirmed that the treated E. Scanning and transmission electron microscopy (SEM and TEM) were used to study the biocidal action of this nanoscale material. These particles were shown to be an effective bactericide. Bacteriological tests were performed in Luria-Bertani (LB) medium on solid agar plates and in liquid systems supplemented with different concentrations of nanosized silver particles. coli was investigated as a model for Gram-negative bacteria. Abstract: The antimicrobial activity of silver nanoparticles against E.


 0 kommentar(er)
0 kommentar(er)
